Department of Chemistry, Fukuoka University of Education
Professor Y. Miyazaki
Our research group is studying the reaction of oxoacids such as boron and vanadium in solutions. We are also working on elucidating the adsorption mechanism of oxoacids onto the selective gels and the state of the adsorbed species.
Thermodynamics of complexation reactions of borate and phenylboronate with diol, triol and tetritol
Boric acid and boronic acid are weak acids, which produce borate or boronate ions in alkaline solutions with changes in the coordination structures of boron from trigonal to tetrahedral. It is well known that the addition of polyols, such as mannitol, is effective for the acid-base titration of boric acid in solutions by a sodium hydroxide solution. Boric acid acts as a stronger acid with the addition of polyol, forming chelate complexes through dehydration condensation between the borate ions and polyols. In order to fully elucidate the structures and stabilities of borate and phenylboronate complexes with polyols, the complexations with 1,2-ethanediol, 1,3-propanediol, 1,2,3-propanetriol, erythritol and threitol were investigated by NMR spectroscopy and DFT calculations. For the borate, 1:1 complexes binding with a ligand in a monodentate manner and 1:2 complexes binding with one ligand in an α,β- or α,γ-bidentate manner and the other in a monodentate manner were identified for the first time besides the well-known 1:1 and 1:2 complexes with five- and/or six-membered chelate rings. In addition, the formations of 1:1 α,β,δ-tridentate complexes with tetritol were confirmed. For the phenylboronate, the bidentate and tridentate complexes with 1:1 stoichiometries were identified. These complexations were enthalpy-driven processes accompanied by a negative entropic contribution. Linear enthalpy−entropy compensation relationships were observed for the stepwise complexations of borate and phenylboronate to form a five- or six-membered chelate ring. 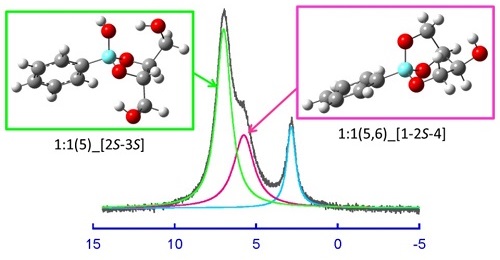 The TΔSº vs. ΔHº plots gave three different straight lines in parallel with one another depending on the number of OH groups outside the chelate ring. For the second intramolecular chelation to form the α,β,δ-tridentate complex from the α,β- or α,γ-bidentate complex with tetritol, the TΔSº vs. ΔHº plots gave different straight lines for the borate and phenylboronate, being more favorable in enthalpy for the phenylboronate.
The TΔSº vs. ΔHº plots gave three different straight lines in parallel with one another depending on the number of OH groups outside the chelate ring. For the second intramolecular chelation to form the α,β,δ-tridentate complex from the α,β- or α,γ-bidentate complex with tetritol, the TΔSº vs. ΔHº plots gave different straight lines for the borate and phenylboronate, being more favorable in enthalpy for the phenylboronate.
Interaction of borate and phenylboronate with the cross-linked dextran gel matrix
Increasing attention has been paid to the environmental toxicity of boron in water. Boron is a micronutrient for plants and animals, but the range between its deficiency and excess is narrow. WHO set a health-based guideline value of 2.4 mg/L for the concentration of boron in drinking water. Selective adsorbents suitable for the effective removal of boron from water is highly demanded.
In this study, the interaction of borate and phenylboronate with dextran gels having different degrees of cross-linking was investigated to clarify the structures of the adsorbed boron species, binding sites of the gel matrix, and the adsorption properties. The main adsorbed species of the phenylboronate was the five-membered mono-chelate complex. In addition to this, the five-membered bis-chelate complex, and the five- and six-membered bis-chelate complex were also the main species for the borate. The boron adsorption was not produced by the glucopyranoside units that were the major constituents of the dextran gel, 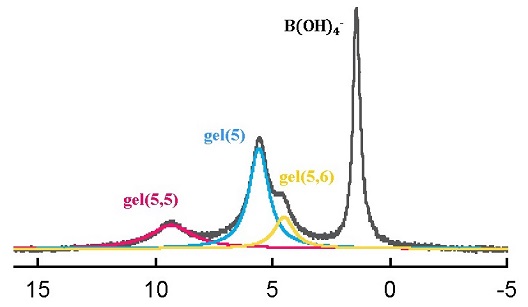 but by the gluconate residues and various forms of the glycerol residues, which had been generated during the cross-linking process of the dextran. The adsorption behavior was reproduced by the calculation taking the complexation of boron with the gel matrix and the Gibbs-Donnan relation into consideration. The knowledge acquired in this study will be useful to establish a new guiding principle to develop boron-selective adsorbents suitable for various purposes.
but by the gluconate residues and various forms of the glycerol residues, which had been generated during the cross-linking process of the dextran. The adsorption behavior was reproduced by the calculation taking the complexation of boron with the gel matrix and the Gibbs-Donnan relation into consideration. The knowledge acquired in this study will be useful to establish a new guiding principle to develop boron-selective adsorbents suitable for various purposes.
Coordination properties of aqueous vanadate complexes with nitrogen- and oxygen-containing multidentate ligands
Tetrahedral vanadate has been physiologically recognized as an analogue of phosphate. The successive protonation from VO43- to H2VO4- is analogous to that from PO43- to H2PO4-. Contrary to phosphate, aqueous vanadate has the flexibility to take different coordination numbers and geometries. The further protonation of the H2VO4- ion ultimately leads to the formation of a six-coordinate cationic species formulated as VO2(H2O)4+. Vanadate interacts with various biogenic substances, such as peptides, nucleosides and nucleotides, and hence, has a specific role in physiological processes. The aqueous vanadate acts as a Lewis acid to accept an electron pair of a ligand, which produces a change in the coordination number from four to five or six. The elucidation of the coordination properties of aqueous vanadate should provide important information about the function of vanadium(V) in biological systems.
In this study, the complexation properties of aqueous vanadate with diethanolamine (DEA) and iminodiacetate (IDA) derivatives were elucidated by NMR spectroscopy and DFT calculations. The coordination geometries of vanadium in 1:1 complexes of the DEA derivatives were confirmed to be trigonal-bipyramidal with a tridentate binding mode. The trigonal-bipyramidal complexes of N,N-bis(2-hydroxyethyl)glycinate (Bicine) and N-(2-hydroxyethyl)iminodiacetate (HIDA) binding through the nitrogen atom, hydroxyethyl and carboxymethyl groups, were also formed at lower and higher pHs, respectively. Vanadate binds with IDA through the nitrogen atom and one of two carboxymethyl groups to form a trigonal-bipyramidal complex, in addition to the trigonal-bipyramidal complex binding through the nitrogen atom and both of the carboxymethyl groups. The latter five-coordinate complex has an open space suitable for additional coordination at the apical position of the central vanadium, which is the same side of the hydrogen atom on the coordinating nitrogen. When an appropriate functional group, such as a carboxymethyl group, acetamido group or hydroxyethyl group, exists instead of the hydrogen atom on the nitrogen, 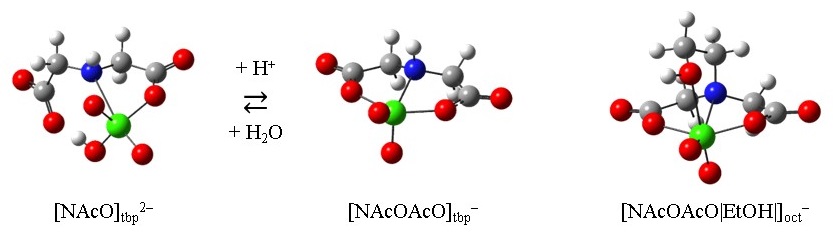 these groups can coordinate to the central vanadium to form a highly stable octahedral complex with a tetradentate binding mode. These findings made it possible to provide an integrated and comprehensive interpretation about the complexation properties of the aqueous vanadate.
these groups can coordinate to the central vanadium to form a highly stable octahedral complex with a tetradentate binding mode. These findings made it possible to provide an integrated and comprehensive interpretation about the complexation properties of the aqueous vanadate.